How Iceland reduced pharmaceutical cold chain waste to less than one percent
Controlant's cold chain origins started in Iceland in the thick of a vaccine crisis. Its impact since then has been global-reaching.
Sign up for the Controlant newsletter
Keep up with developments in pharma supply chain visibility. Sign up for Controlant Insights and receive our latest white paper.
Responding to a global epidemic
Controlant’s origins grew out of a collaborative project started by its co-founders at the University of Iceland more than a decade ago. As graduate students, they were developing new wireless sensor technologies that could automatically register temperature monitoring data and send it to a centralized cloud database.
In 2009, the H1N1 virus, or “Swine Flu,” began to spread throughout Asia, causing global concerns regarding public health and safety. Iceland’s Directorate of Health and Icelandic Medicines Agency acquired large quantities of the H1N1 vaccine to protect the country’s population.
Concerned that the storage coolers located throughout Iceland were not reliable enough to store the vaccine supplies, the Directorate of Health sought out a new solution that could be promptly implemented.
Within two weeks, Controlant’s real-time temperature and humidity monitoring solutions were installed within several dozens of refrigeration units in 52 health clinics throughout Iceland to monitor vaccine conditions for supplies that needed to be kept between 2-8°C. This ensured product quality, integrity, and safety for end patients. Eventually, the technology and services were also implemented to protect the vaccines while en route to their intended destinations.
Conditions of clinic refrigerators
Upon analyzing the coolers at the clinic sites, it was discovered that at least 30 percent of the vaccine storage units were maintaining conditions that exceeded the recommended boundaries and may have been for years. This could have potentially impacted the integrity of vaccines delivered to patients.
When deploying the Controlant solution, it became evident that the conditions of the refrigerators varied. For instance, their 24-hour mean temperature varied, on average, from 1.5°C to 12.3°C. Additionally, 18 percent of the refrigerators held a 24-hour-mean temperature that exceeded the required temperature limits. 32 percent of the refrigerators exceeded temperature boundaries for one hour within a 24-hour period, and 51 percent were going beyond the given limits for more than 10 minutes within a 24-hour period.
Implementing a 24/7 vaccine monitoring solution
Controlant worked closely with the Icelandic agency to implement its real-time cold chain monitoring solution in accordance with the relevant standards and requirements. Internet of Things (IoT) tracking devices were installed within all 59 refrigerators to track temperature and humidity conditions in real-time, continuously sending data to Controlant's cloud-enabled software platform. Due to the simplicity of the system, the setup process and user configuration took only a few minutes within each clinic.
Employees at each health clinic now have on-demand access to real-time and historical temperature and humidity data through the Controlant portal. If environmental conditions begin to deviate beyond the designated parameters, notifications and alerts are automatically visible within the software platform's dashboard and are sent to designated stakeholders via email and SMS so that proactive response can be taken to prevent vaccine and pharmaceutical waste.
Reducing cold chain waste to less than one percent
At the time of the Controlant roll-out, a number of refrigerators were replaced with new units where it was evident they weren't keeping temperatures within the required 2-8°C boundaries.
Within twelve months, vaccine spoilage resulting from temperature excursions within clinics throughout Iceland was nearly eliminated. Figures 1 and 2 below demonstrate the temperature improvements within the vaccine storage units during the first two months after implementation of the Controlant solution.
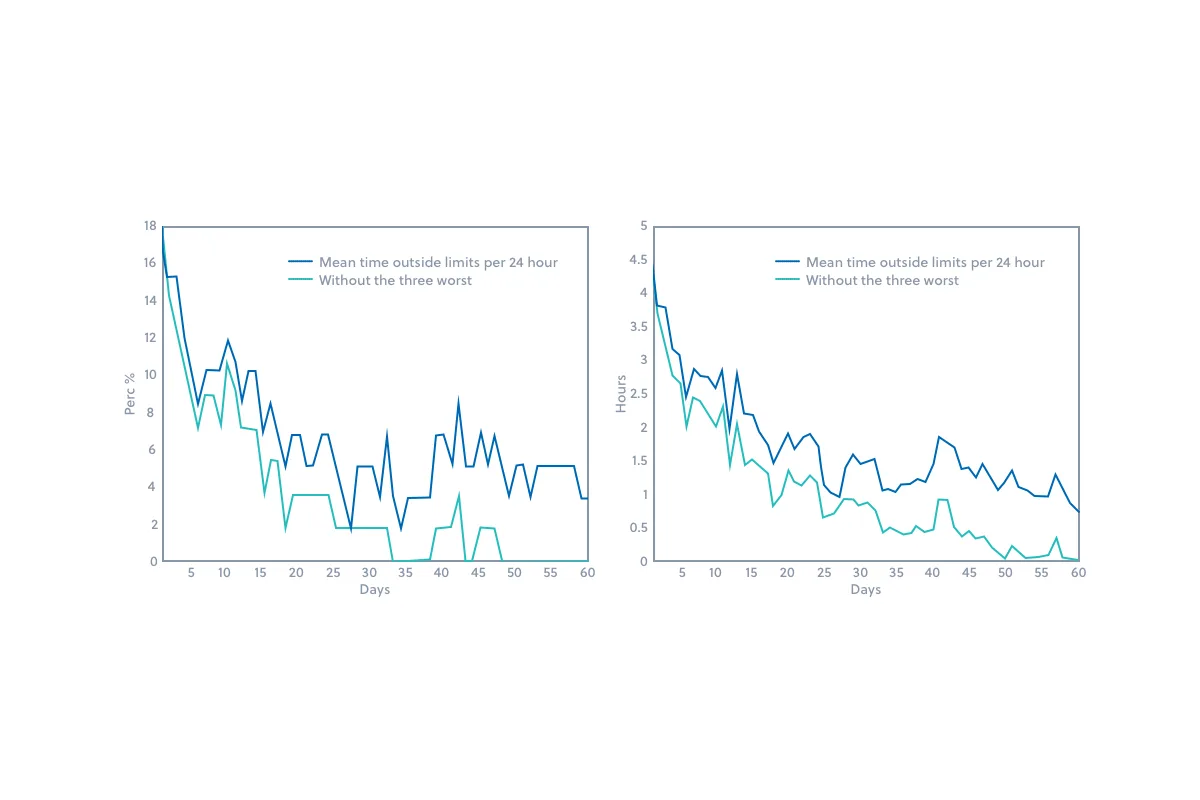
Figure 1: The left graph shows the percentage of refrigerators with a 24-hour mean temperature that exceeded the established boundaries. The right graph shows the mean time within a 24-hour period during which the refrigerators exceeded the established temperature boundaries.
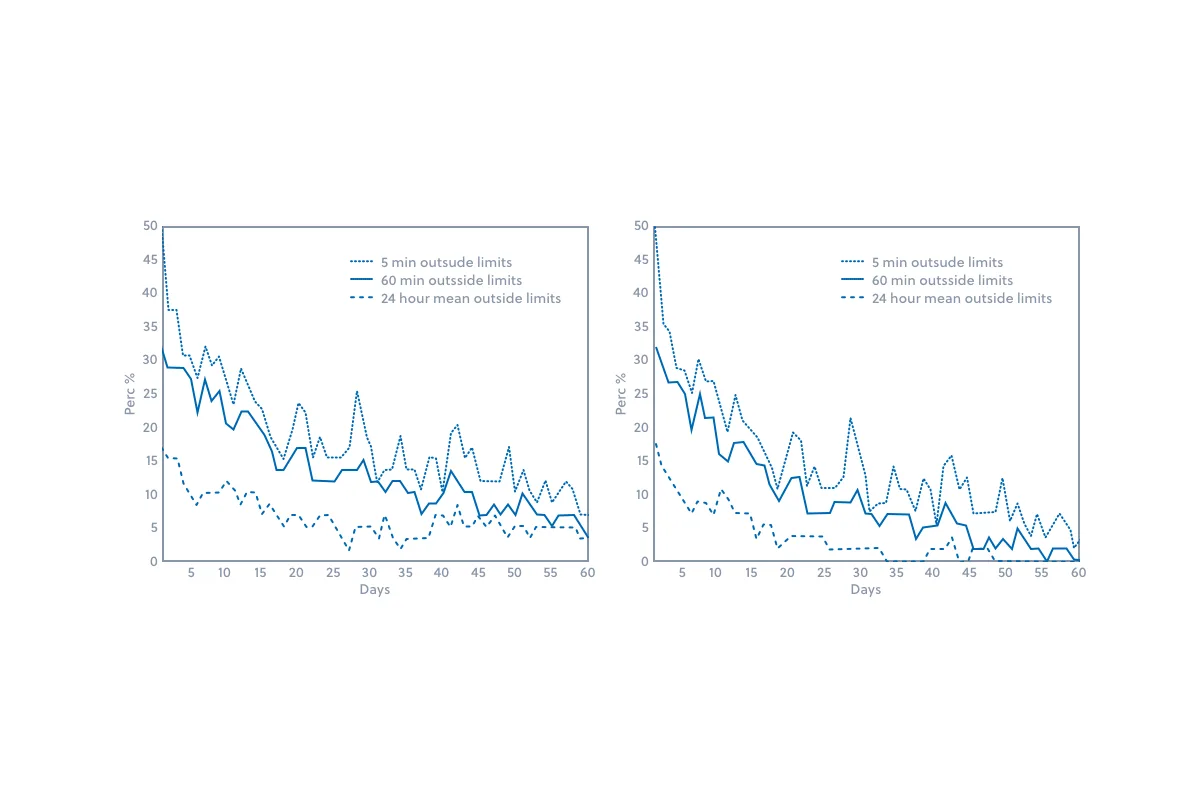
Figure 2: The above graphs demonstrate the percentage of refrigerators that held temperatures beyond the required boundaries after implementation of the Controlant solution. The left graph includes all refrigerator units and the right graph excludes the 3 worst performing units.
During the initial two-month implementation period, more than 100 temperature alerts were issued, with nearly 40 percent of them occurring outside regular working hours. Root cause analyses conducted using the Controlant data led to the conclusion that the primary reasons for the deviations were:
- Adjustments were needed to the refrigeration units to keep the vaccine conditions safe
- Doors to the refrigeration units were often left open
- Heavy usage (opening and closing doors) by staff affected the inside temperature conditions
- Local power failures inside the clinics were resulting in the refrigeration units losing their temperature
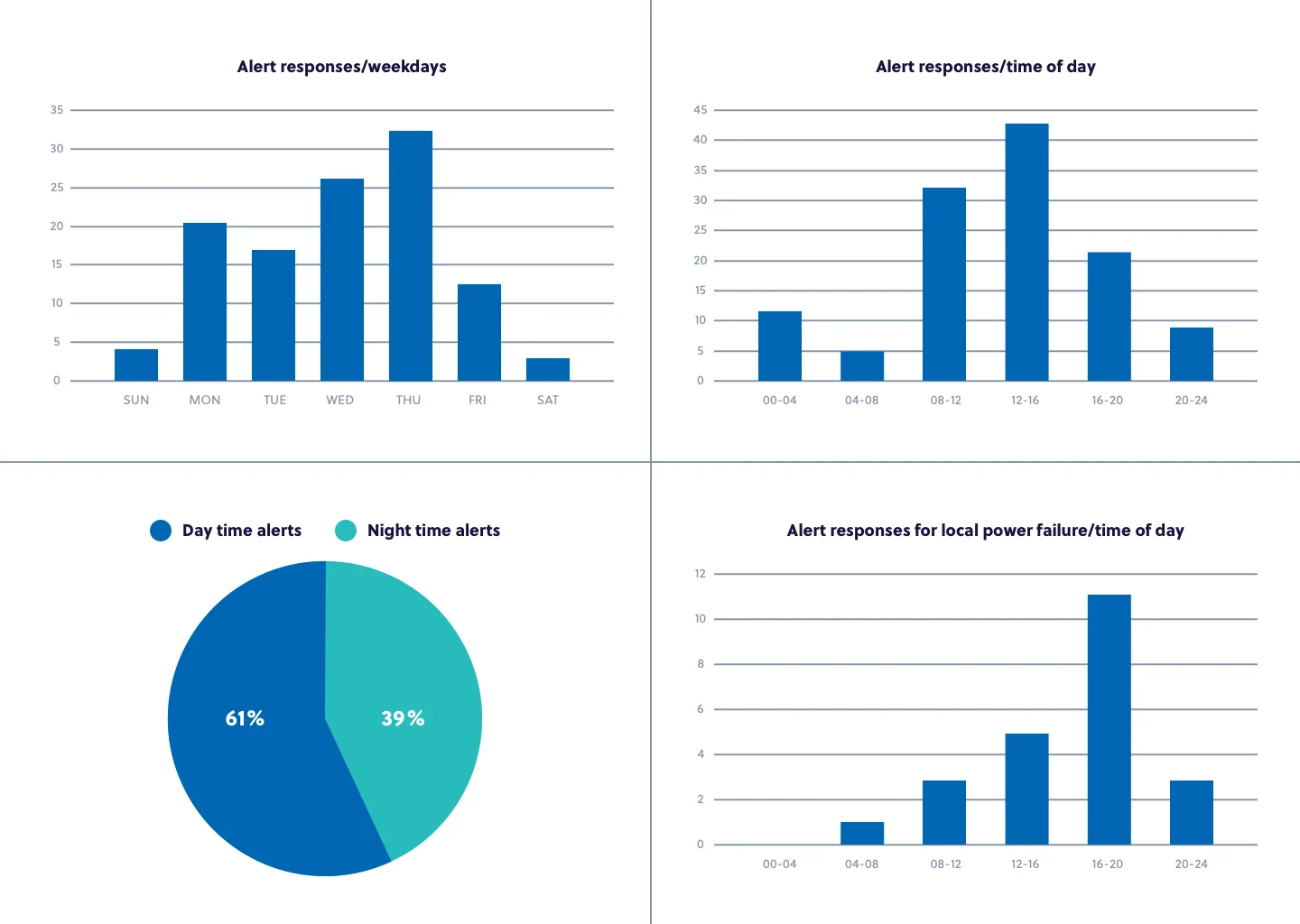
Armed with these actionable insights, the clinics were able to take corrective action to mitigate further risks and drive continuous improvements to protect vaccine and medicine supply. Within two months, considerable improvements in temperature and stability were achieved due to data sharing and collaboration among stakeholders throughout the Icelandic agency and among clinic staff.
Over time, cold chain vaccine and medicine waste throughout Iceland has been reduced to 0.3 percent through the implementation of Controlant's real-time supply chain visibility solution inside clinics, pharmacies, and hospitals as well as the culture change that has resulted from the proactive, 24/7 cold chain monitoring service.
Inspiring a global mission
"Our co-founders have children, including myself," said Gisli Herjolfsson, CEO. "A cold chain breakdown could impact the integrity of vaccines that were administered to any of them. Knowing that after the implementation of our continuous environmental monitoring solution, vaccine integrity could be safely maintained helped to assure us that our children's health could be sufficiently protected. We wanted to extend that peace of mind to others."
The proactive cold chain control achieved in Iceland led Controlant’s founding team to its current mission to reduce global cold chain waste due to temperature excursions to less than one percent. Controlant's unique model — Cold Chain as a Service®— combines its IoT environmental tracking devices measuring product conditions while they are in transit and in storage, a proprietary, cloud-enabled software platform continuously delivering real-time and prescriptive analytics and insights, and a variety of managed and professional services, including 24/7 monitoring and response, and IoT asset management and reverse logistics. All are delivered as an on-demand, subscription-based service.
Since 2009, the company has partnered with top global pharmaceutical enterprises and food chains to protect their end users, improve their supply chain performance, automate the quality review process, and reduce waste through proactive risk mitigation and insights that help drive continuous improvements.


